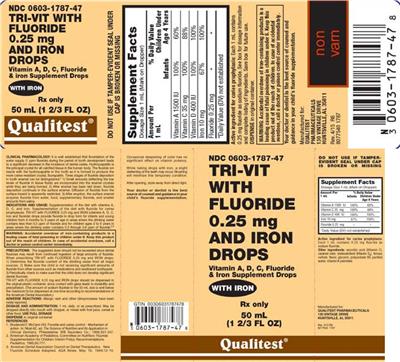
Tri-Vit With Fluoride 0.25 mg and Iron Drops
Product Overview
Brand: Qualitest
Barcode: 3 0603-1785-47 4
Net Contents: 50 mL; 1.6907 fl. Oz.
Serving Size (Overview): 1 mL [Mark on Dropper]
Product Type: Multi-Vitamin and Mineral (MVM) [A1315]
Form: Liquid [E0165]
Date Entered: Nov 21, 2020
Market Status: On Market
Suggested Use (Overview): See box for dosage information and complete listings of ingredients. Save box for future use. Dispense in original container. Indications and Usage: Tri-Vit with Flouride 0.25 mg drops may be useful for infants whose diets are lacking in vitamins A; D and C. Tri-Vit with Flouride 0.25 mg drops also provide fluoride for caries prophylaxis. Tri-Vit with Flouride 0.25 mg (vitamins A; D; C; and fluoride) drops were developed to provide fluoride in drop form for infants and young children from 6 months to 3 years of age in areas where the drinking water contains less than 0.3 ppm of fluoride and for children ages 3 to 6 years in areas where the drinking water contains 0.3 through 0.6 ppm of fluoride. Tri-Vit with Fluoride 0.25 mg drops should be dispensed in the original plastic container; since contact with glass leads to instability and precipitation. (The amount of sodium fluoride in the 50-mL size is well below the maximum to be dispensed at one time according to recommendations of the American Dental Association.) Dosage and Administration: 1 mL daily; or as prescribed. May be dropped directly into mouth with dropper; or mixed with fruit juice; cereal or other food. Use full dosage. Dispense in original container
Product Self-Roles:
- No specific self-roles defined.
Dietary Supplement Facts
| Ingredient | Category | Amount Per Serving | Unit | % Daily Value | Target Group | Serving Size (Fact) |
|---|---|---|---|---|---|---|
| Vitamin A | vitamin | 1500 | IU | 100 | Infants | 1 mL |
| Vitamin A | vitamin | 1500 | IU | 60 | Children less than 4 years of age | 1 mL |
| Vitamin C | vitamin | 35 | mg | 100 | Infants | 1 mL |
| Vitamin C | vitamin | 35 | mg | 88 | Children less than 4 years of age | 1 mL |
| Vitamin D | vitamin | 400 | IU | 100 | Infants | 1 mL |
| Vitamin D | vitamin | 400 | IU | 100 | Children less than 4 years of age | 1 mL |
| Iron | mineral | 10 | mg | 67 | Infants | 1 mL |
| Iron | mineral | 10 | mg | 100 | Children less than 4 years of age | 1 mL |
| Fluoride | mineral | 0.25 | mg | N/A | Infants | 1 mL |
| Fluoride | mineral | 0.25 | mg | N/A | Children less than 4 years of age | 1 mL |
Other Ingredients
Each 1 ml contains (Fluoride)
Label Statements
Statement of Identity
- Vitamin A D C Fluoride & iron Supplement Drops
Other
- Rx only Your doctor or dentist is the best source of counsel and guidance in your child's fluoride supplementation. N Clinical Pharmacology: It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries. Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal fluorapatite. Three stages of fluoride deposition in tooth enamel can be distinguished: 1) Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed. 2) After enamel has been laid down fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted. 3) After eruption the surface enamel acquires fluoride from water food supplementary fluoride and smaller amounts from saliva. References1. Brudevold F McCann HG. Fluoride and caries control; Mechanism of action. In: Nizel AE ed. The Science of Nutrition and Its Application in Clinical Dentistry. Philadelphia: WB Saunders Co.; 1966;331-347.2. American Academy of Pediatrics Committee on Nutrition: Fluoride Supplementation for Children: Interim Policy Recommendations. Pediatrics. 1995;95(5):777.3. American Dental Association Council on Dental Therapeutics. New Fluoride Schedule Adopted. ADA News. May 16 1994;12-14.
Precautions
- Do not use if tamper-evident seal under cap is broken or missing Warning: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose call a doctor or poison control center immediately. Precautions: The suggested dose should not be exceeded since dental fluorosis may result from continued ingestion of large amounts of fluoride. When prescribing Tri-Vit with Fluoride 0.25 mg drops: 1) Determine the fluoride content of the drinking water from all major sources. 2) Make sure the child is not receiving significant amounts of fluoride from other sources such as medications and swallowed toothpaste. 3) Periodically check to make sure the child does not develop significant dental fluorosis. Adverse Reactions: Allergic rash and other idiosyncrasies have been rarely reported. While taking drops with iron a slight darkening of the teeth may occur. Brushing will minimize this temporary condition.
Formulation
- Active ingredient for caries prophylaxis:Each 1 mL contains 0.25 mg fluoride as sodium fluoride. Occasional deepening of color has no significant effect on vitamin potency.
Suggested Use
- See box for dosage information and complete listings of ingredients. Save box for future use. Dispense in original container. Indications and Usage: Tri-Vit with Flouride 0.25 mg drops may be useful for infants whose diets are lacking in vitamins A D and C. Tri-Vit with Flouride 0.25 mg drops also provide fluoride for caries prophylaxis. Tri-Vit with Flouride 0.25 mg (vitamins A D C and fluoride) drops were developed to provide fluoride in drop form for infants and young children from 6 months to 3 years of age in areas where the drinking water contains less than 0.3 ppm of fluoride and for children ages 3 to 6 years in areas where the drinking water contains 0.3 through 0.6 ppm of fluoride. Tri-Vit with Fluoride 0.25 mg drops should be dispensed in the original plastic container since contact with glass leads to instability and precipitation. (The amount of sodium fluoride in the 50-mL size is well below the maximum to be dispensed at one time according to recommendations of the American Dental Association.) Dosage and Administration: 1 mL daily or as prescribed. May be dropped directly into mouth with dropper or mixed with fruit juice cereal or other food. Use full dosage. Dispense in original container
Product Specific Information
- After opening store away from direct light.
Associated Company Information & Roles
Qualitest Pharmaceuticals
Huntsville,
AL
35811
USA
Roles for this Product:
- Distributor